In 1906, the founder of the Richard Wolf , a German instrument maker named Georg Wolf, introduced his finely crafted surgical instruments at a small medical conference in Berlin.
Over the next decade, with these surgical instruments, Wolf and his craftsmen became the most famous endoscopes manufacturers at that time.
From the very beginning, Wolf strongly believed that his devices would become the future of surgery.
With strong pioneering spirit, over 100 years of establishment and development, Richard Wolf has become a global leader in minimally invasive surgery, which provides solutions to improve surgical efficiency and opens a new breakthrough in surgical procedures.
Now Richard Wolf’s headquarter is in Knittlingen, Germany with 16 subsidiaries and more than 130 sales agents worldwide.
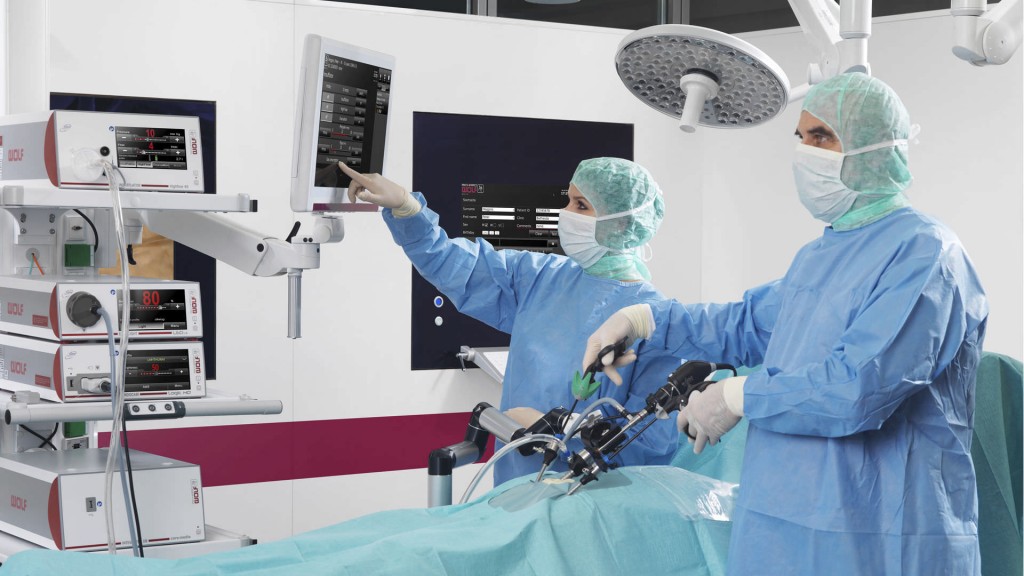
The company’s main products are laparoscopic imaging systems, auxiliary machines for laparoscopic surgery and endoscopic surgical instruments in specialties such as: abdominal cavity, obstetrics, urology, thorax business, ENT, joint etc. and many other technological applications such as integrated operating room, shock wave treatment system, laser lithotripsy system and endoscopic products in the aviation industry.
Following the philosophy “Approaching perfection” and take the target “Product and service quality is the foundation to ensure customer success and satisfaction” as the 1st concern, Richard Wolf has constantly focused on improving, innovating and doing product research and development to achieve excellent quality and highest safety.
Richard Wolf’s quality management system is certified in accordance with international standards such as DIN EN ISO 13485 and EC Directive 93/42/EEC. Being an international company, Richard Wolf also meets the international requirements of other countries around the world. In addition, Richard Wolf is certified MDSAP (Medical Device One-Time Reduced-Evaluation Program).
Richard Wolf’s manufacturing process is carried out in a high-tech facility, which perfectly combines the mechanical, precision engineering, electronics and software. All products have passed a certified quality control system, so customers can fully believe in Richard Wolf’s product quality.
“Made in Germany. A promise that we keep every day – with hearts, minds and hands. ”
TNT Medical is very proud to be a distributor of Richard Wolf’s products. If you need any support about products & service, please contact us for more information.




Leave A Comment